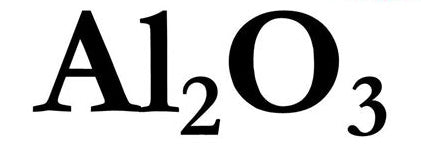 The Activated Alumina refining process is old. Also named "Aluminum Oxide" The craft dates back to the 1880s when the Bayer company developed it for the aluminum industry. They crush up "Bauxite" (a raw material mined from the Earth) and mix it with sodium hydroxide.
The Activated Alumina refining process is old. Also named "Aluminum Oxide" The craft dates back to the 1880s when the Bayer company developed it for the aluminum industry. They crush up "Bauxite" (a raw material mined from the Earth) and mix it with sodium hydroxide.
It's then seeded with crystals to generate aluminum hydroxide. The hydroxide is then heated in a kiln to remove any water leaving several versions of granular, powdered alumina that includes smelter-grade alumina, activated alumina, and calcined alumina.

Typically, "Activated Alumina" can be in granular form or a uniform "beaded" (shown above) substance that is porous and used as a substrate for catalysts and as an adsorbent for removing water from gases and liquids.
Smelter-grade alumina accounts for 90 percent of all the alumina produced; it's transported to aluminum plants, electrolyzed and formed into aluminum metal.
Calcined alumina is made into a variety of ceramic products, including spark-plug insulators, integrated-circuit packages, bone and dental implants, laboratory ware, sandpaper grits and grinding wheels, and refractory linings for industrial furnaces.
These products exhibit the well known alumina properties, including low electric conductivity, resistance to chemical attack, high strength, extreme hardness (9 on the Mohs hardness scale, the highest rating being 10), and high melting point (approximately 2,050 °C, or 3,700 °F).
These products exhibit the well known alumina properties, including low electric conductivity, resistance to chemical attack, high strength, extreme hardness (9 on the Mohs hardness scale, the highest rating being 10), and high melting point (approximately 2,050 °C, or 3,700 °F).
It's an ideal desiccant for use in large air drying systems and water filtration facilities.
Alumina Manufacturing Process


